
The two bonds to substituents A in the structure on the left are of this kind. As defined in the diagram on the right, a simple straight line represents a bond lying approximately in the surface plane. In most cases the focus of configuration is a carbon atom so the lines specifying bond directions will originate there. In order to represent such configurations on a two-dimensional surface (paper, blackboard or screen), we often use perspective drawings in which the direction of a bond is specified by the line connecting the bonded atoms. This shape is dependent on the preferred spatial orientation of covalent bonds to atoms having two or more bonding partners.Three dimensional configurations are best viewed with the aid of models. The remaining two hybrid orbitals containing a lone pair of the electron remains nonbonded.The three dimensional shape or configuration of a molecule is an important characteristic. The two half-filled (containing unpaired electron) sp3 hybrid orbitals of oxygen overlap axially with two half-filled 1s orbitals of two hydrogen atoms separately to form two O-H bonds (sigma bond). Thus geometry is angular or V-shaped, in which the oxygen lies at the centre, two hydrogen atoms occupy two corners of the tetrahedron and lone pair of electrons occupy remaining two corners of the tetrahedron. The two half-filled (containing unpaired electron) sp 3 hybrid orbitals of oxygen overlap axially with two half-filled 1s orbitals of two hydrogen atoms separately to form three O-H bonds. Thus water has distorted the tetrahedral shape. But due to greater repulsion exerted by lone pair on bonding orbitals, the angle is reduced to 104.5 0. All N-H bonds in ammonia are of equal strength.įour sp3hybridised orbitals formed, repel each other and they should be directed towards the four corners of a regular tetrahedron and the angle between them should be 109.5°. The bonds between Nitrogen and hydrogen are sp3- s. The fourth hybrid orbital containing lone pair of the electron remains non bonded. The three half-filled (containing unpaired electron) sp3 hybrid orbitals of nitrogen overlap axially with three half-filled 1s orbitals of three hydrogen atoms separately to form three covalent N-H bonds (sigma bonds). The three half-filled (containing unpaired electron) sp3 hybrid orbitals of nitrogen overlap axially with three half-filled 1s orbitals of three hydrogen atoms separately to form three covalent N-H bonds Hence geometry is tetrahedral pyramidal in which the nitrogen lies at the centre, three hydrogen atoms form the base and one pair of electrons forms the apex of the pyramid. Thus ammonia has distorted tetrahedral shape. But due to greater repulsion exerted by lone pair on bonding orbitals, the angle is reduced to 107°. The other three orbitals are half-filled and they are bonding orbitals.
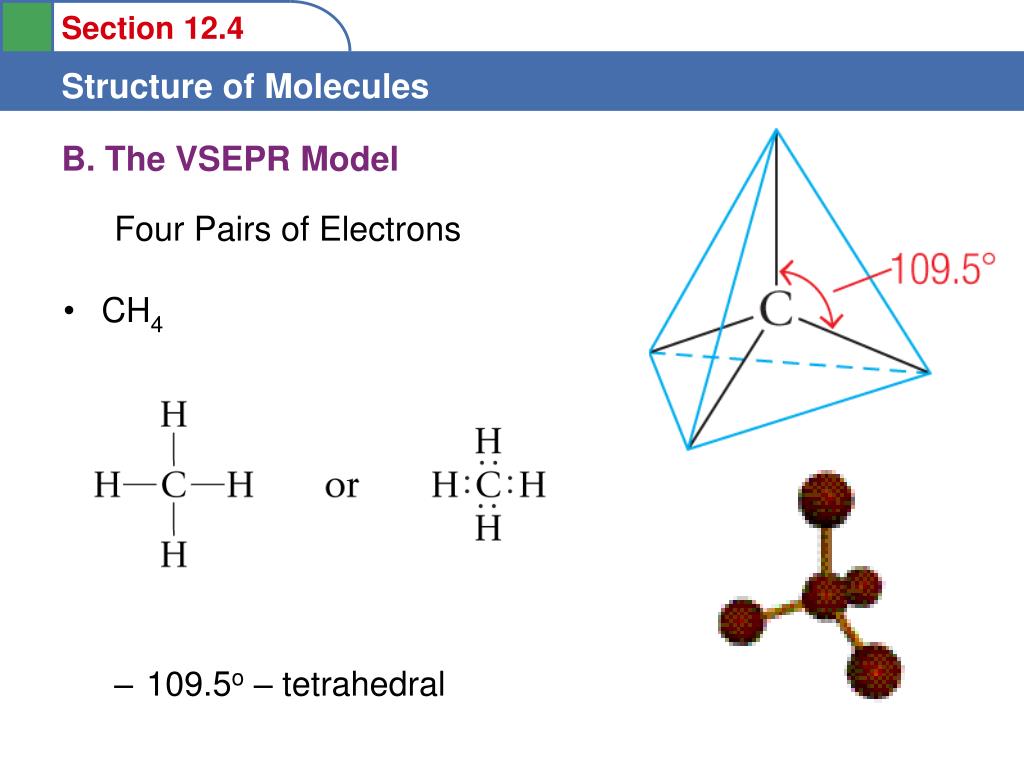
One hybrid orbital has paired electrons and it is nonbonding orbital. All C-H bonds in methane are of equal strength.įour sp 3 hybridized orbitals formed, repel each other and they should be directed towards the four corners of a regular tetrahedron and the angle between them should be 109.5°. The bonds between carbon and hydrogen are sp 3– s. H-C-H bond angle is 109.5°.įour sp 3 hybrid orbitals of carbon atom having one unpaired electron each overlap separately with 1s orbitals of four hydrogen atom along the axis forming four covalent bonds (sigma bonds). With a carbon atom at the centre and four hydrogens at the four corners of a Thus in CH 4 molecule has a tetrahedral structure Separately with 1s orbitals of four hydrogen atom along the axis forming fourĬovalent bonds. Hybrid orbitals of carbon atom having one unpaired electron each overlap Due to this maximum overlapping is achieved. Only a bigger lobe is involved in bond formation. In each sp 3 hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Each sp 3 hybrid orbital contains one unpaired electron. Orbitals formed, repel each other and they are directed towards the fourĬorners of a regular tetrahedron.


 0 kommentar(er)
0 kommentar(er)
